Honors and Awards
Excellent doctoral thesis supervisor of Tsinghua University (2021, 2018)
Thieme Chemistry Journals Award (2019)
The First Prize of Science and Technology Award of Chinese Pharmaceutical Society (2019)
The Second Prize of National Natural Science Award (2016)
Guangdong Province Science and Technology Progress Award (2015)
Tsinghua-Bayer Investigator Award (2014)
Tsinghua-Janssen Investigator Award (2011-2015)
Research Interests
The major research interests in the Tang’s laboratory could be defined as “Biological Function-Oriented Chemical Synthesis” (Fig. 1). Simply, the primary goal of our research is to discover and develop new reactions and synthetic strategies which enable the rapid access of biologically important molecules (particularly natural products and heterocyclic compounds), and then apply them to relevant biomedical studies. Currently, the major research directions in our laboratory include: (1) total synthesis of natural products that have unique molecular architecture, important biological profile and novel biosynthetic pathway; (2) development of new synthetic methods which enable the rapid access of privileged structures in natural products and drugs; (3) design, synthesis and biological evaluation of small molecules as anti-viral, anti-tumor and neuroprotective agents, and (4) development new bioorthogonal reactions and their application in chemical biology.
Major Scientific Achievements
1).Guided by the research concept of “biosynthetic inspiration combined with rational design”, we have solved the synthetic challenges associated with multiple prominent families of natural products including xanthanolides, cytochalasans, plakortin-type polyketides and Stemona alkaloids (Fig. 2). With more than 60 natural targets having been completed to date, this part of work has paved the way to further explore their biological functions and medicinal value.
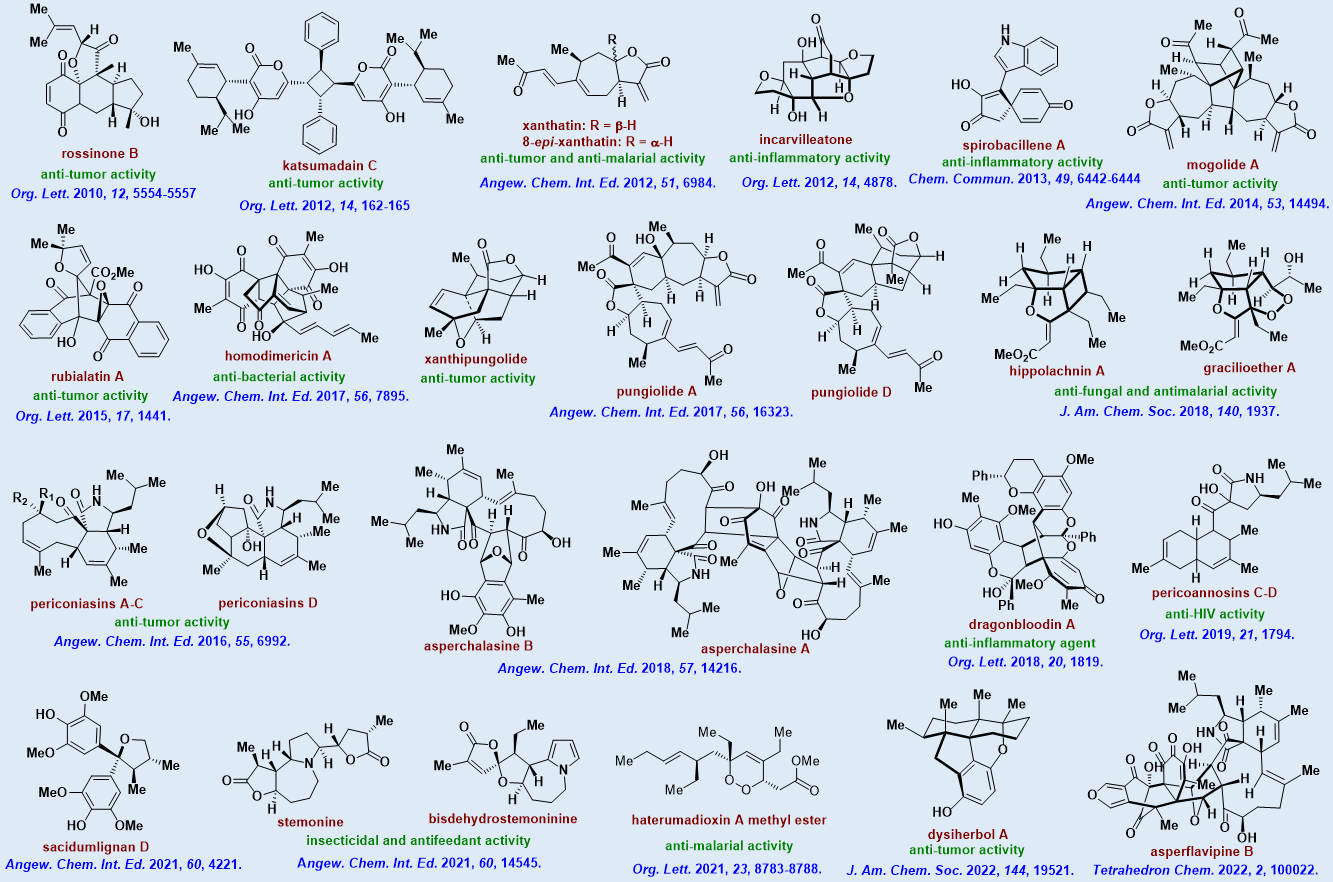
Figure 2:Total syntheses of natural products completed by the Tang’s group
2).Focusing on two subjects of “ring-strain-driven rearrangements and ring-expansion reactions” and “ring-opening chemistry of triazoles”, we have developed a series of novel and powerful synthetic methodologies (Fig. 3), which enable the rapid construction of various privileged structures of natural products or drugs, thereby providing the key technical support for total synthesis and drug discovery.
Figure 3:Synthetic methodologies based on the ring-opening chemistry of triazoles
3).Based on inspiration from natural product synthesis, we have established several unique ring- strain-driven bioorthogonal reactions (Fig. 4), some of which have been successfully applied to in vitro protein labeling and live cell imaging.
Figure 4: Development of nature-inspired bioorthogonal reactions
4).We have developed two classes of small molecules with great potential as drug candidates, including a class of tryptophanyl-tRNA synthetase (TrpRS) inhibitors with anti-Mycobacterium tuberculosis activity (Fig. 5) and a class of nicotinamide mononucleotide adenosyltransferase (NMNAT) agonists with neuroprotective effect (Fig. 6).
Figure 5: Development of novel anti-tuberculosis agent
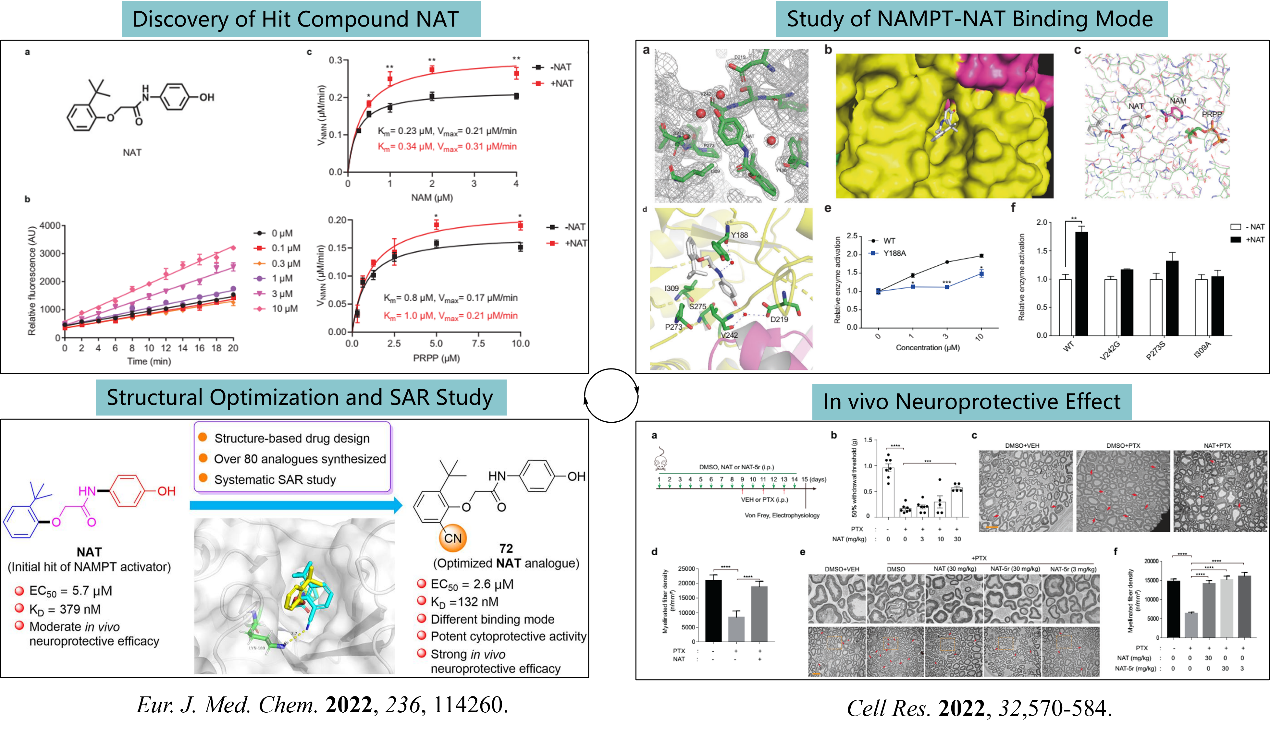
Figure 6: Development of novel NAMPT activators
Selected Publications
1. Hu, S. K.;
Tang, Y. F. Enantioselective Total Synthesis of Dysiherbols A, C, and D. J. Am. Chem. Soc.
2022, 144, 19521-19531.
2. Yao, H.#; Liu, M. H.#; Wang, L. B.#; Zu, Y. M.#; Wu, C.; Li, C. Y.; Zhang, R. X.; Lu, H. G.; Li, F. F.; Xi, S.; Chen, S. Q.; Gu, X. Y.; Liu, T. Y.; Cai, J.; Wang, S. R.; Yang, M. J.; Hua, L.; Xing, G. G.;
Tang, Y. F.*; Wang, G. L.* Discovery of small-molecule activators of nicotinamide phosphoribosyltransferase (NAMPT) and their preclinical neuroprotective activity. Cell Res.
2022, 32, 570-584.
3. Bao, R. Y.; Zhang, H. Y.;
Tang, Y. F.* Biomimetic Synthesis of Natural Products: A Journey To Learn, To Mimic, and To Be Better. Acc. Chem. Res.
2021, 54, 3720–3733.
4. Guo, Z.; Bao, R. Y.; Li, Y. H.; Li, Y. S.; Zhang, J. Y.;
Tang, Y. F.* Tailored Synthesis of Skeletally Diverse Stemona Alkaloids through Chemoselective Dyotropic Rearrangements of β-Lactones. Angew. Chem. Int. Ed.
2021, 60, 14545–14553.
5. Lei, X. Q.; Li, Y. H.; Lai, Y.; Hu, S. K.; Qi, C.; Wang, G. L.*;
Tang, Y. F.* Strain-Driven Dyotropic Rearrangement: A Unified Ring-Expansion Approach to α-Methylene-γ-butyrolactones. Angew. Chem. Int. Ed.
2021, 60, 4221–4230.
6. Yang, H. Z.; Zeng, T. Y.; Xi, S.; Hu, S. K.; Wu, Y. F.;
Tang, Y. F. Photo-induced, Strain-promoted Cycloadditions of trans-Cycloheptenones and Azides. Green Chemistry,
2020, 22, 7023-7030.
7. Bao, R. Y.; Tian, C.; Zhang, H. Y.; Wang, Z. G.; Dong, Z.; Li, Y. H.; Gao, M. H.; Zhang, H. L.; Liu, G.*;
Tang, Y. F.* Total Syntheses of Asperchalasines A–E. Angew. Chem. Int. Ed.
2018, 57, 14216-14220.
8. Li, Q. G.#; Zhao, K.#; Peuronen, A.; Rissanen, K.; Enders, D.*;
Tang, Y. F.* Enantioselective Total Syntheses of (+)-Hippolachnin A, (+)-Gracilioether A, (-)-Gracilioether E and (-)-Gracilioether F. J. Am. Chem. Soc.
2018, 140, 1937–1944.
9. Feng, J.; Lei, X. Q.; Bao, R. Y.; Li, Y. H.; Xiao, C. Q.; Hu, L. H.;
Tang, Y. F.* Enantioselective and Collective Total Syntheses of Xanthanolides. Angew. Chem. Int. Ed.
2017, 56, 16323–16327.
10. Feng J.#; Lei, X. Q.#; Guo, Z.;
Tang, Y. F.* Total Synthesis of Homodimericin A. Angew. Chem. Int. Ed.
2017, 56, 7895–7899.
11. Wang, Y. F.; Wu, Y. F.; Li, Y. H.;
Tang, Y. F.* Denitrogenative Suzuki and Carbonylative Suzuki Coupling Reactions of Benzotriazoles with Boronic Acids. Chem. Sci.
2017, 8, 3852–3857.
12. Tian, C.; Lei, X. Q.; Wang, Y. H.; Dong, Z.; Liu, G.*;
Tang, Y. F.* Total Syntheses of Periconiasins A-E. Angew. Chem. Int. Ed.
2016, 55, 6992–6996.
13. Shang, H.#; Liu, J. H.#; Bao, R. Y.; Cao, Y. Zhao, K.; Xiao, C. Q.; Zhou, B.; Hu, L. H.*;
Tang, Y. F.* Biomimetic Synthesis: Discovery of Xanthanolide Dimers. Angew. Chem. Int. Ed.
2014, 53, 14494–14498.
14. Shang, H.#; Wang, Y. H.#; Tian, Y. Feng, J.;
Tang, Y. F.* The Divergent Synthesis of Nitrogen Heterocycles by Rhodium(II)-Catalyzed Cycloadditions of 1-Sulfonyl 1,2,3-Triazoles with 1,3-Dienes. Angew. Chem. Int. Ed.
2014, 53, 5662–5666.
15. Fu, J. K.; Shang, H.; Wang, Z. F.; Chang, L.; Shao, W. B.; Yang, Z.*;
Tang Y. F.* Gold-Catalyzed Rearrangement of Allylic Oxonium Ylides: Efficient Synthesis of Highly Functionalized Dihydrofuran-3-ones. Angew. Chem. Int. Ed.
2013, 52, 4198–4202.
16. Ren, W. W.; Bian, Y. C.; Zhang, Z. Y.; Shang, H.; Zhang, P. T.; Chen, Y. J.; Yang, Z.*; Luo T. P.*;
Tang Y. F.* Enantioselective and Collective Syntheses of Xanthanolides by Controllable Dyotropic Rearrangement of cis--Lactones. Angew. Chem. Int. Ed.
2012, 51, 6984–6988.
17. Xiao, Q.#; Ren W. W.#; Chen, Z. X.; Sun, T. W.; Li, Y.; Ye, Q. D.; Gong, J. X.; Meng, F. K.; You, L.; Liu, Y. F.; Zhao, M. Z.; Xu, L. M.; Shan, Z. H.; Shi, Y.;
Tang, Y. F.*; Chen, J. H.*; Yang Z.* Diastereoselective Total Synthesis of (+)-Schindilactone A. Angew. Chem. Int. Ed.
2011, 50, 7373–7377.
18. Nicolaou, K. C.*; Wang, J. H.;
Tang, Y. F.; Botta, L. Total Synthesis of Sporolide B and 9-epi-Sporolide B. J. Am. Chem. Soc.
2010, 132, 11350–11363.
19. Nicolaou, K. C.*;
Tang, Y. F.; Wang, J. H. Total Synthesis of Sporolide B. Angew. Chem. Int. Ed.
2009, 48, 3501–3505.
20. Nicolaou, K. C.*;
Tang, Y. F.; Wang, J. H. Total Synthesis and Antibacterial Properties of Carbaplatensimycin. J. Am. Chem. Soc.
2007, 129, 14850-14851.


