Research Interests:
The abnormal stem cells in diseases could cause aberrant regeneration, bad quality of wound healing and tissue repair, defects in the physiological functions, and disease recurrence and tumor development. Therefore, the study of adult stem cells under various pathological conditions is absolutely fundamental and critical to address the pathogenesis, promote tissue self-remodeling and identify new therapeutic approaches for human diseases in the field of regenerative medicine.
The primary focus of Wang’s laboratory is to study adult stem cells in digestive system diseases. Currently, the major research areas in Wang’s laboratory include but not limited to: 1) Identify the properties and alterations in IBD patient-derived intestinal stem cells under normal and disease conditions, and try to cure or recover the “disease stem cells” by small molecules or genetic and epigenetic modifications; 2) Study the heterogeneity of colorectal cancer using cultured patient-derived single-cancer-cell expanded pedigrees, 3) establish novel digestive disease model using adult stem cells to provide insights into the pathogenic mechanisms and to find prospective candidate drugs and therapeutic strategies for patients; 4)Investigate the properties of liver adult stem cells derived from Liver fibrosis/Cirrhosis patients . 5)Study pancreatic adult stem cells from diabetes patients, as well as the relationship between ductal stem cells and pancreatic ductal adenocarcinoma. 6) Long-term Culture of Spinal cord neural stem cells and differentiation of functional neurons for the application of stem cell therapy and regenerative medicine.
Patient-specific stem cell pedigrees could be used as the basis and source to identify drug sensitivity patterns and to perform high-throughput drug screening for targeted therapies and precision medicine.
Scientific Contributions:
Prof. Wang has been working on adult stem cells and human diseases. She and her colleagues discovered that a discrete population of cells at the junction of the esophagus and stomach is the cellular origin of Barrett’s metaplasia. This novel mechanism underlying the etiology of Barrett’s Esophagus transformed people’s review in early diagnosis and preventive therapy targeting cancer precursors of esophageal cancer. This finding was published in Cell as a featured article. Additionally, Prof. Wang has established a technology platform in cloning ground-state epithelial stem cells that can be propagated robustly and faithfully in vitro. It has been demonstrated that these stem cells, after long-term culture, still have the long-term self-renewal capacity and could maintain precise lineage commitment. Moreover, she also established a 3D culture system that can precisely recapitulate intestinal epithelium architecture in vitro and showed that this system can be applied to model intestinal diseases, such as pseudomembranous colitis (Wang X. et al., Nature, 2015). Furthermore, she and her colleagues have applied this technology to clone and study the stem cells in Barrett’s Esophagus, esophagus and gastric epithelium using patient-matched endoscopic biopsies. The molecular genetics and transformation of these distinct stem cell pedigrees provide insights into the origin, progression, and possibly the therapeutic eradication of these precursor lesions. This work was published in Nature Communications.
Selected Achievements
1). A hypothetical opportunistic migration of junctional cells toward adjacent damaged squamous cells following chronic acid reflux, which shows the cellular origins of Barrett’s metaplasia.
Figure 1. Schematic diagram shows junctional origins of Barrett’s metaplasia following chronic acid reflux in humans. (Wang X. et al., Cell, 2011)
2). A novel technology for columnar epithelial stem cells using 3T3-J2 cells as feeder layer and cocktail of factors (including R-Spondin 1, Jagged-1, Noggin, Rock inhibitor, TGF-beta receptor inhibitor, Nicotinamide etc.). Single-cell-derived stem cell pedigrees could be expanded in vitro with properties of long-term self-renewal and stable lineage commitment.
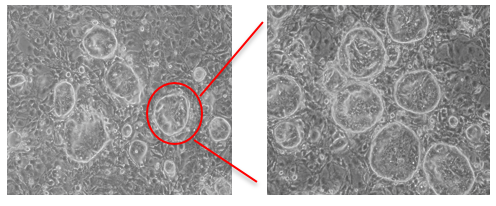
Figure 2. Intestinal stem cell colonies from pool (left) and pedigree (right) growing on 3T3-J2 feeder layer (Wang X. et al., Nature, 2015)
3). A novel air-liquid interface (ALI) culture system for the differentiation of columnar epithelial stem cells into 3D epithelial tissue structure with intact barrier function (Wang X. et al., Nature, 2015).
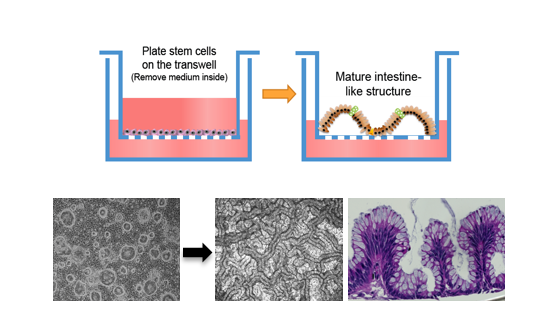
Figure 3. 3D intestinal epithelial tissue derived from stem cells in the ALI culture system.
Honors and Awards
Bayer Investigator (2018)
Bayer Investigator (2017)
The Thousand Talents Plan (Youth) (2016)
Harvard Chinese Life Science Annual Distinguished Research Award (2012)
Zhu Liyuehua Outstanding Doctoral Award of Chinese Academy of Sciences (2009)
Outstanding graduate student of Institute of Genetics and Developmental Biology Chinese Academy of Sciences (2009)
Outstanding Thesis of Institute of Genetics and Developmental Biology Chinese Academy of Sciences (2009)
President Scholarship of Chinese Academy of Sciences (2005)
Selected Publications:
1. Zhao Y, Zhang B, Ma Y, Zhao F, Chen J, Wang B, Jin H, Zhou F, Guan J, Zhao Q, Wang H, Liu Q#, Zhao F#, Wang X# Colorectal Cancer Patient-derived 2D and 3D Models Efficiently Recapitulate Inter- and Intra-tumoral Heterogeneity. Advanced Science. 2022 (DOI: 10.1002/advs.202201539)
2. Yamamoto Y*, Wang X*(*Co-first authors), Bertrand D, Kern F, Zhang T, Deluba M, Srivastava S, Khor CC, Hu YY, Wilson L, Blaszyk H, Rolshud D, Ming T,Liu JJ, Howitt B, Vincent M, Crum CP, Nagarajan N, Ho KY, McKeon F, and Xian W. Mutational spectrum of Barrett’s stem cells suggests paths to initiation of a precancerous lesion. Nature Communications. 2016. doi: 10.1038/ncomms10380.
3. Yamamoto Y, Ning G, Howitt B, Mehra K, Wu LY, Wang X, Hong Y, Kern F, Nagarajan N, Torti S, Brewer M, Choolani M, McKeon F, Crum CP and Xian W. In Vitro and In Vivo Correlates of Physiologic and Neoplastic Human Fallopian Tube Stem Cells. Manuscript in revision. The Journal of Pathology. 2016. 238:519-530.
4. Wang X*, Yamamoto Y*(*Co-first authors), Wilson LH, Zhang T, Howitt B, Ning G, Hong Y, Khor CC, Chevalier B, Bertrand D, Nagarajan N, Sylvester FA, Farrow MA, Lacy DB, Ho KY, Crum CP, McKeon F and Xian W. Cloning and variation of ground state intestinal stem cells. Nature. 2015. 522(7555):173-178.
5. Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. P63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015. 517(7536): 616-20.
6. Ning G, Bijron JG, Yamamoto Y, Wang X, Howitt BE, Herfs M, Yang E, Hong Y, Cornille M, Wu L, Hanamornroongruang S, McKeon FD, Crum CP, Xian W. The PAX2-null immunophenotype defines multiple lineages with common expression signatures in benign and neoplastic oviductal epithelium. The Journal of Pathology. 2014. 234(4): 478-87.
7. Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon F, Xian W, and Crum CP. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. PNAS. 2012. 109(26): 10516–10521.
8. Gao, Y., Wei, J., Han, J., Wang, X., Su, G., Zhao, Y., Chen, B., Xiao, Z., Cao, J., and Dai, J. The Novel Function of OCT4B Isoform-265 in Genotoxic Stress. Stem Cells. 2012. 30(4):665-72.
9. Wang X*, Ouyang H*, Yamamoto Y*, (*Co-first authors), Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, Crum CP, Xian W, McKeon F. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell. 2011. 145(7):1023-35. Featured Article in Cell. Highlighted in Nature Reviews Cancer. Faculty 1000 Biology: 10 (Exceptional).
10. Wang, X and Dai, J. Isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010. 28(5):885-93. (Invited Review)
11. Gao, Y., Wang, X. *(* Co-first authors), Han, J., Xiao, Z., Chen, B., Su, G., Dai, J. The novel OCT4 spliced variant OCT4B1 can generate three protein isoforms by alternative splicing into OCT4B. J Genet Genomics. 2010. 37(7):461-5
12. Zhang, J., Wang, X. *(* Co-first authors), Chen, B., Xiao, Z., Li, W., Lu, Y. and Dai, J. The human pluripotency gene NANOG/NANOGP8 is expressed in gastric cancer and associated with tumor development. Oncology Letters. 2010. 1(3):457-463.
13. Zhang, W., Wang, X., Xiao, Z., Liu, W., Chen, B. and Dai, J. Mapping of the minimal internal ribosome entry site element in the human embryonic stem cell gene OCT4B mRNA. Biochemical and Biophysical Research Communications. 2010. 394(3):750-4
14. Zhao, Y., Zhang, J., Wang, X., Chen, B., Xiao, Z., Shi, C., Wei, Z., Hou, X., Wang, Q. and Dai, J. The osteogenic effect of bone morphogenetic protein-2 on the collagen scaffold conjugated with antibodies. Journal of Controlled Release. 2010. 141(1):30-7.
15. Wang, X., Zhao, Y., Xiao, Z., Chen, B., Wei, Z., Wang, B., Zhang, J., Han, J., Gao, Y., Li, L., Zhao, H., Zhao, W., Lin, H. and Dai, J. Alternative translation of OCT4 by an internal ribosome entry site and its novel function in stress response. Stem Cells. 2009. 27(6):1265-75.
16. Zhang J., Ding L., Zhao Y., Sun W., Chen B., Lin H., Wang X., Zhang L., Xu B. and Dai J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation. 2009. 119(13): 1776-84
17. Zhang, J.*, Wang, X.* (* Co-first authors), Han, J., Chen, B., Wang, B., Suo, G. and Dai J. NANOGP8 is a retrogene expressed in cancers. FEBS Journal. 2006. 273(8): 1723-30.
18. Zhang, J., Wang, X., Zhao, Y., Chen, B., Suo, G. and Dai J. Neoplastic transformation of cultured human fibroblasts after long-term serum starvation.Cancer Letters. 2006. 243: 101-108.
19. Suo, G., Han, J., Wang, X., Zhang, J., Zhao, Y., Zhao, Y. and Dai, J. Oct4 pseudogenes are transcribed in cancers. Biochemical and Biophysical Research Communications. 2005. 337, 1047-1051.
20. Zhang, J., Wang, X., Chen, B., Suo, G., Zhao, Y., Duan, Z. and Dai J. Expression of nanog gene promotes NIH3T3 cell proliferation. Biochemical and Biophysical Research Communications. 2005. 338: 1098-1102.


