Research Interests
Many fundamental biological processes such as the sense of touch and pain and the regulation of vascular development and blood pressure require designated mechanotransducers for converting mechanical force into electrochemical signals, a process termed mechanotransduction. Mechanically activated (MA) cation channels represent a specialized type of mechanotransducers for rapidly responding to changes of mechanical force to either excite cell membranes or trigger biochemical signaling. However, the molecular identities and mechanogating mechanisms of mammalian MA cation channels remain largely unknown, which has rendered mechanotransduction much less understood compared to our understanding of chemotransduction mediated by ligand-gated ion channels and electrotransduction mediated by voltage-gated ion channels. Following the discovery and establishment of the MA Piezo channel family, we have been utilizing Piezo1 and Piezo2 as prototypes of mammalian MA cation channels to understand how Piezo channels effectively convert mechanical force into selective cation permeation at an atomic-level of spatial resolution, a millisecond-level of temporal resolution, a picoampere-level of current resolution and a piconewton level of force resolution, and how their mechanosensitivity and ion permeation properties precisely control various mechanotransduction processes at both cellular and animal levels. Our ultimate goal is to harness the deep understanding of Piezo channels for developing novel therapeutics for disease treatment or novel technologies for biological manipulation. Toward these research aims, we have established a multidisciplinary research platform covering molecular biology, biochemistry, structural biology, cell biology, patch-clamp electrophysiology, high-throughput drug screening and mouse and human genetics, and made significant progress in understanding the structure-function relationship and physiological and pharmacological regulations of Piezo channels.
Current Research Focus
Combining our established research platform and expertise in studying MA ion channels and innovative methodologies for drug discovery and novel ion channel identification, we aim to:
1) understand the dynamic mechanogating mechanism of Piezo channels;
2) discover and develop drugs targeting Piezo channels;
3) explore how the biophysical properties of Piezo channels determine their physiological and pathophysiological roles in vivo;
4) identify and characterize novel MA channels via utilizing an innovative structural and pharmacological profiling approach.
The proposed studies might not only advance our understanding of MA ion channels and mechanotransduction mechanisms, but also help to reveal novel therapeutic targets for treating devastating diseases such as pain, bone loss, heart diseases, and cancer.
Major Scientific Contributions
• Determined the cryo-EM structures of the full-length mouse Piezo1, a novel Piezo1 isoform-Piezo1.1 identified by my lab, and Piezo2, revealing their unique structural features and providing structural blueprints for uncovering their ion permeation, mechanogating and regulatory mechanisms (Nature 2015; Nature 2018; Nature 2019; Neuron 2020).
• Identified the bona fide pore with three intracellular lateral portal-based ion-conducting routes, the transmembrane gate, the intracellular lateral plug gates, and key pore property-determining residues, elucidating the ion permeation mechanism (Neuron 2016; Neuron 2020).
• Identified key mechanogating components and pathways and proposed the plug-and-latch gating, dual-gating and lever-like gating models for underlying the mechanogating mechanisms of Piezo channels (Nature 2018; Nature Communications 2018; Neuron 2020).
• Identified Piezo interacting proteins and alternative splicing variants, providing insight into physiological regulatory mechanisms (Nature Communications 2017; Neuron 2020; BioRxiv 2020).
• Identified Piezo1 activators Jedi1 and Jedi2 via establishing a high-throughput drug-screening platform and revealed their activation mechanism (Nature Communications 2018).
• Explored the role of Piezo channels in determining the physiological and pathophysiological processes of touch and pain sensation, bone formation and heart function (Cell Reports 2019; eLife 2019; Nature Communications 2021).
• Demonstrated that STIM1 functions as an in vivo warm sensor in keratinocytes to define the optimal preference temperature (OPT), which might be utilized as a peripheral reference temperature for precise warm sensation (Cell Research 2019).
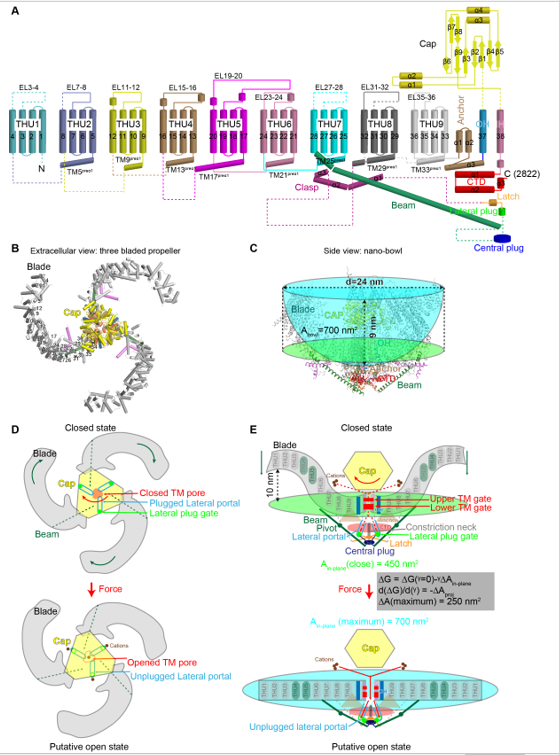
Figure Structural design and mechanogating mechanisms of Piezo channels.
A, The 38-TM topology comprised of nine repetitive transmembrane helical units (THUs) of 4-TMs each, the outer helix (OH), the pore-lining inner helix (IH), and other featured domains such as the Beam, Lateral Plug, Latch, the intracellular C-terminal domain (CTD), Anchor, and the extracellular Cap. B, The extracellular view of the three bladed propeller structure. C, The side view of the nano-bowl configuration shaped by the highly curved transmembrane regions of the three blades is illustrated in the cyan bowl. The mid-plane opening diameter, depth, surface area (Abowl) and the in-plane membrane area (Ain-plane of 450 nm2) shown in green oval are labeled.
D, Force-induced rotation of the Cap (indicated by the orange arrow) and the blades (indicated by the dark green arrows) might open the transmembrane pore and unplug the lateral plug gates to allow cation conduction via the central transmembrane pore and the three intracellular lateral portals, which are labeled in the top panel. E, When the blades become completely planar upon membrane tension application (indicated by the dark green arrows), the surface area of the nano-bowl will be expanded to a maximal in-plane membrane area of 700 nm2. In the grey box, tension (γ) induced free energy change upon transition from closed to open channel is described by the free energy equation. The tension sensitivity is determined by the change of the membrane area expansion (ΔAin-plane), which might reach a maximal value of 250 nm2. The dashed and solid red arrows in the top and bottom panels respectively indicate the closed and open ion-conducting pathways. Panel D and E also illustrate the dual-gating model of Piezo channels, in which the transmembrane gate is gated by motion of the top Cap, while the lateral plug gates are coordinately unplugged via a plug-and-latch mechanism upon flattening the curved blade-membrane system. THU4 and THU5 are shown in dark green to highlight their reported role in mechanogating of Piezo1.
Honors and Awards
The National Science Fund for Distinguished Young Scholar
The 10,000 Talent Program Scholar
The Young 1,000 Talent Program Scholar
Excellent Young Scientists Award
Bayer Investigator Fellowship and Janssen Investigator Fellowship in Tsinghua University
Selected Publications
1. Yao Rong*, Jinghui Jiang*, Yiwei Gao*, Jianli Guo, Danfeng Song, Wenhao Liu, Mingmin Zhang, Yan Zhao#,
Bailong Xiao#, Zhenfeng Liu# (2021) TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells.
Elife. 2021 Aug 19;10:e71474. doi: 10.7554/eLife.71474. Online ahead of print.
2. Yang Jiang*, Xuzhong Yang*, Jinghui Jiang*,
Bailong Xiao# (2021) Structural Designs and Mechanogating of the Mechanosensitive Piezo channels.
Trends Biochem Sci. 2021 Feb 17:S0968-0004(21)00022-0. doi: 10.1016/j.tibs.2021.01.008.
3. Fan Jiang, Kunlun Yin, Kun Wu, Mingmin Zhang, Shiqiang Wang, Heping Cheng, Zhou Zhou,
Bailong Xiao# (2021) The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction.
Nature
Commun. 2021 Feb 8;12(1):869. doi: 10.1038/s41467-021-21178-4.
4. Jing Wang*, Jinghui Jiang*, Xuzhong Yang, Li Wang,
Bailong Xiao# (2020) Tethering Piezo channels to the actin cytoskeleton for mechanogating via the E-cadherin-β-catenin mechanotransduction complex.
bioRxiv preprint posted May 13, 2020. doi: https://doi.org/10.1101/2020.05.12.092148.
5. Jie Geng*, Wenhao Liu*, Heng Zhou*, Tingxin Zhang*, Li Wang*, Mingmin Zhang, Yiran Li, Bo Shen, Xueming Li
#,
Bailong Xiao# (2020) A plug-and-latch mechanism for gating the mechanosensitive Piezo channel.
Neuron. 2020 May 6;106(3):438-451.e6.doi: 10.1016/j.neuron.2020.02.010 (Featured by
Neuron Previews).
6. Bailong Xiao
# (2020) Levering mechanically activated Piezo channels for potential pharmacological intervention.
Annual Review of Pharmacology and Toxicology 2020 Jan 6;60:195-218. doi: 10.1146/annurev-pharmtox-010919-023703.
7. Li Wang
*, Heng Zhou
*, Mingmin Zhang
*, Wenhao Liu
*, Tuan Deng, Qiancheng Zhao, Yiran Li, Jianlin Lei, Xueming Li
#,
Bailong Xiao
# (2019) Structure and mechanogating of the mammalian tactile channel PIEZO2.
Nature
(Article) 2019 Sep;573(7773):225-229. doi: 10.1038/s41586-019-1505-8. Epub 2019 Aug 21. (Featured by
Nature News & Views).
8. Weijia Sun
*, Shaopeng Chi
*, Yuheng Li, Shukuan Ling, Yingjun Tan, Youjia Xu, Fan Jiang, Jianwei Li, Caizhi Liu, Guohui Zhong, Dengchao Cao, Xiaoyan Jin, Dingsheng Zhao, Xingcheng Gao, Zizhong Liu,
Bailong Xiao#, and Yingxian Li
# (2019) The mechanosensitive Piezo1 channel is required for bone formation.
Elife. 2019 Jul 10;8. pii: e47454. doi: 10.7554/eLife.47454.
9. Mingmin Zhang, Yanfeng Wang, Jie Geng, Shuqin Zhou,
Bailong Xiao
# (2019) Mechanically Activated Piezo Channels Mediate Touch and Suppress Acute Mechanical Pain Response in Mice.
Cell Rep. 2019 Feb 5;26(6):1419-1431.e4. doi: 10.1016/j.celrep.2019.01.056.
10. Xiaoling Liu*, Haiping Wang*, Yan Jiang*, Qin Zheng*, Matt Petrus, Mingmin Zhang, Sisi Zheng, Christian Schmedt, Xinzhong Dong,
Bailong Xiao
# (2019) STIM1 thermosensitivity defines the optimal preference temperature for warm sensation in mice.
Cell Res. 2019 Jan 3. doi: 10.1038/s41422-018-0129-0.
11. Qiancheng Zhao*, Heng Zhou*, Xueming Li
#,
Bailong Xiao# (2018) The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism.
FEBS J. 2019 Jul;286(13):2461-2470. doi: 10.1111/febs.14711. Epub 2018 Dec 14.
12. Qiancheng Zhao*, Heng Zhou*, Shaopeng Chi*, Yanfeng Wang*, Jianhua Wang, Jie Geng, Kun Wu, Wenhao Liu, Tingxin Zhang, Meng-Qiu Dong, Jiawei Wang, Xueming Li
#,
Bailong Xiao# (2018) Structure and Mechanogating Mechanism of the Piezo1 Channel.
Nature (Article) 2018 Feb 22;554(7693):487-492. doi: 10.1038/nature25743. Epub 2018 Jan 22. (Featured by
Nature News & Views; Selected by
Faculty of 1000.)
13. Yanfeng Wang*, Shaopeng Chi*, Huifang Guo, Guang Li, Li Wang, Qiancheng Zhao, Yu Rao, Liansuo Zu, Wei He,
Bailong Xiao# (2018) A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel.
Nat Commun. 2018 Apr 3;9(1):1300. doi: 10.1038/s41467-018-03570-9.
14. Yubo Wang,
Bailong Xiao# (2018). The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction.
J Physiol. 2018 Mar 15;596(6):969-978.
15. Tingxin Zhang*, Shaopeng Chi*, Fan Jiang, Qiancheng Zhao,
Bailong Xiao# (2017). A protein interaction mechanism for suppressing the mechanosensitive Piezo channels.
Nat Commun. 2017 Nov 27;8(1):1797.
16. Jie Geng*, Qiancheng Zhao*, Tingxing Zhang*,
Bailong Xiao# (2017). In Touch With the Mechanosensitive Piezo Channels: Structure, Ion Permeation and Mechanotransduction.
Curr Top Membr. 2017;79:159-195.
17. Qiancheng Zhao*, Kun Wu*, Jie Geng*, Shaopeng Chi*, Yanfeng Wang, Peng Zhi, Mingmin Zhang,
Bailong Xiao# (2016). Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels.
Neuron. 2016 Mar 16;89(6):1248-63. (Previewed by
Neuron; Reported by www.Eurekalert.org)
18. Jingpeng Ge*, Wanqiu Li*, Qiancheng Zhao*, Ningning Li*, Maofei Chen, Peng Zhi, Ruochong Li, Ning Gao
#,
Bailong Xiao#, Maojun Yang
# (2015). Architecture of the mammalian mechanosensitive Piezo1 channel.
Nature (Article). Nov 5;527(7576):64-9.
19. Bertrand Coste* (co-first),
Bailong Xiao*
(co-first), Jose S. Santos, Ruhma Syeda, Jorg Grandl, Kathryn S. Spencer, Sung Eun Kim, Manuela Schmidt, Jayanti Mathur, Adrienne E. Dubin, Mauricio Montal, Ardem Patapoutian (2012). Piezo Proteins Are Pore-forming Subunits of Mechanically Activated Channels.
Nature. 2012 Feb 19;483(7388):176-81 PMID:22343900 (
Highly cited paper and this study has been cited by the textbook of Principals of Neurobiology edited by Dr. Liqun Luo; Selected by
Faculty of 1000; Featured by
Nature News & Views; TSRI News & Views; Reported by Science Daily)
20. Bailong Xiao*, Bertrand Coste, Jayanti Mathur, Ardem Patapoutian (2011). Temperature-dependent STIM1 activation induces Ca
2+ influx and modulates gene expression.
Nat Chem Biol. 2011 Jun;7(6):351-8. PMID: 21499266 (
Nominated for 2011 signaling breakthroughs of the year by Science Signaling; Featured by
Nat Chem Biol. News & Views; Featured by TSRI News & Views; Reported by Science Daily)


